Vial Technology Platform
Vial is a clinical
research organization (CRO) delivering faster and more efficient trials for sponsors through its
end-to-end technology platform. To deliver on this vision our team built 5 customer facing products
and several internal facing applications. Below I have shortly outlined the most important steps of
the
process for some of the platforms I worked on.
The product design team was made of 3 designer, one Senior Product Designer (Me), the Head of Design, and one Product Designer.
The product design team was made of 3 designer, one Senior Product Designer (Me), the Head of Design, and one Product Designer.
- UI/UX
- Visual design
- Prototyping
- Research
- Design QA
- Brand Identity
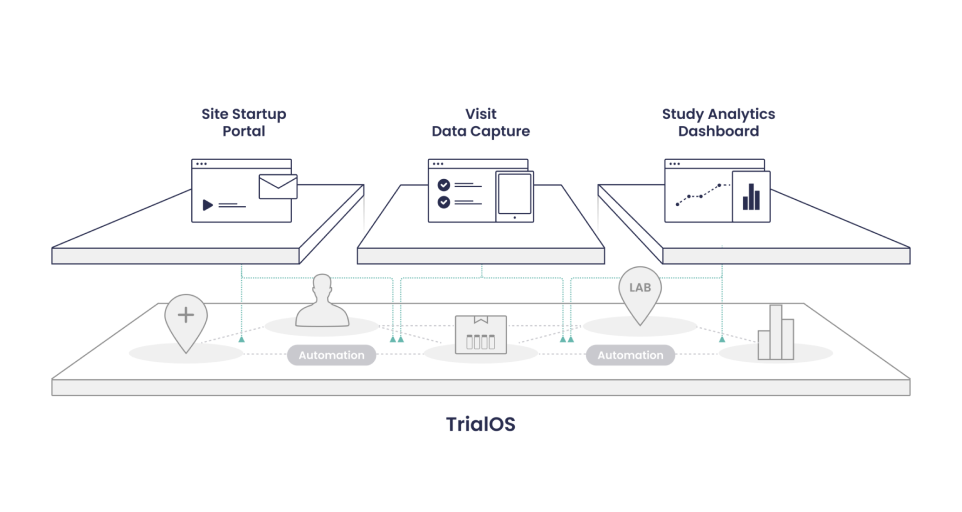

1. Electronic Data Capture (EDC)
Electronic Data
Capture (EDC) is admittedly a fairly generic sounding term, but in the clinical trials
field it actually means something fairly specific: using systems to collect clinical trial data in
electronic form as opposed to paper form. This was the first and most important application we were
tasked with building.
The EDC is a critical component of modern clinical research, streamlining the data collection
process
and enhancing efficiency, accuracy, and data quality. They help researchers, sponsors, and
regulatory
authorities manage trial data effectively.
As such, one thing was clear from the beginning, this system would be utilized by multiple
stakeholders
involved in clinical research and trials.
1.1 Role and Process
As a team of
initally 2
designers, we individually led the design on different projects. By working closely together, and
with
our product manager and engineers on the team, we were able to have a very open and collaborative
process.
I was a very strong advocate for integrating more user research throughout all stages of the design
process, resulting in:
- A design requirements document (‘DRD’) that would be created before a project kick-off and shared with the team alongside the existing product requirements doc. The DRD gathered and grouped user research from all available sources and framed the core issues/needs within the overarching jobs-to-be-done for the product. This document helped to bring the team onto the same page, empathize with our users and align on priorities from a user experience perspective.
- Remote usability testing practice with clinical staff that helped us test designs and prototypes more frequently and consistently and allowed the whole team to be involved.


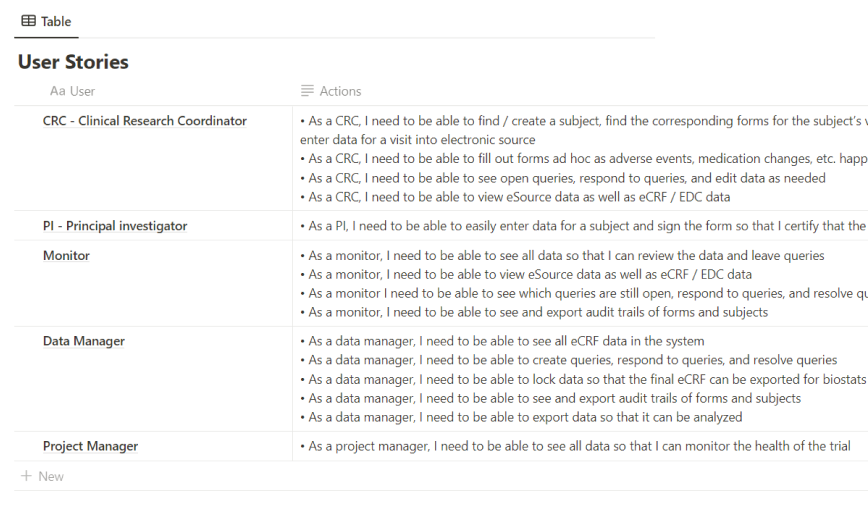
1.2 Understanding Users and User Needs
Understanding the
requirements of clinical researchers, data managers, and end-users was foundational. The product and
design team conducted thorough research involving a mix of qualitative and quantitative methods,
including interviews, surveys, and observing user workflows.
It was essential to grasp the pain points, challenges, and specific needs of each user group. Researchers would prioritize ease of data entry and access, while data managers would focus on data integrity and system integrations. Gathering this comprehensive understanding helped in creating a system that met diverse user needs.
It was essential to grasp the pain points, challenges, and specific needs of each user group. Researchers would prioritize ease of data entry and access, while data managers would focus on data integrity and system integrations. Gathering this comprehensive understanding helped in creating a system that met diverse user needs.
.png)
1.3 Conceptualization and Ideation
Once user needs
were identified, the conceptualization phase began. This involved brainstorming sessions and
collaborative workshops to generate initial concepts and wireframes. Sketches and rough prototypes
helped visualize the system's structure and functionalities. While rapid prototypes aided in
creating clickable prototypes that allowed for early-stage testing and validation.


1.4 Design System & UI Design
Having in mind
that this would be just the first application of many that we were going to be working on, it was
obvious that creating a design system early on was going to be very important in order to
ensure
visual and functional consistency across apps, efficiency, scalability, as well as ease of
maintainability and cross-team collaboration.
Creating a user-friendly interface was paramount, as researchers and data managers will spend significant time interacting with the system. Iterative design refinements based on usability testing and feedback sessions were conducted to fine-tune the interface. This involved surveys and phone calls with clinical staff that validated some of our design and product assumptions.
After many iterations and rounds of user testing and phone calls to validate that we were making the right changes, we launched in an internal Beta period to gather more feedback from our staff. Post-launch, I followed up on the phone with many of the staff that had provided initial feedback, and we also launched a feedback widget for users to provide feedback in-product.
Creating a user-friendly interface was paramount, as researchers and data managers will spend significant time interacting with the system. Iterative design refinements based on usability testing and feedback sessions were conducted to fine-tune the interface. This involved surveys and phone calls with clinical staff that validated some of our design and product assumptions.
After many iterations and rounds of user testing and phone calls to validate that we were making the right changes, we launched in an internal Beta period to gather more feedback from our staff. Post-launch, I followed up on the phone with many of the staff that had provided initial feedback, and we also launched a feedback widget for users to provide feedback in-product.

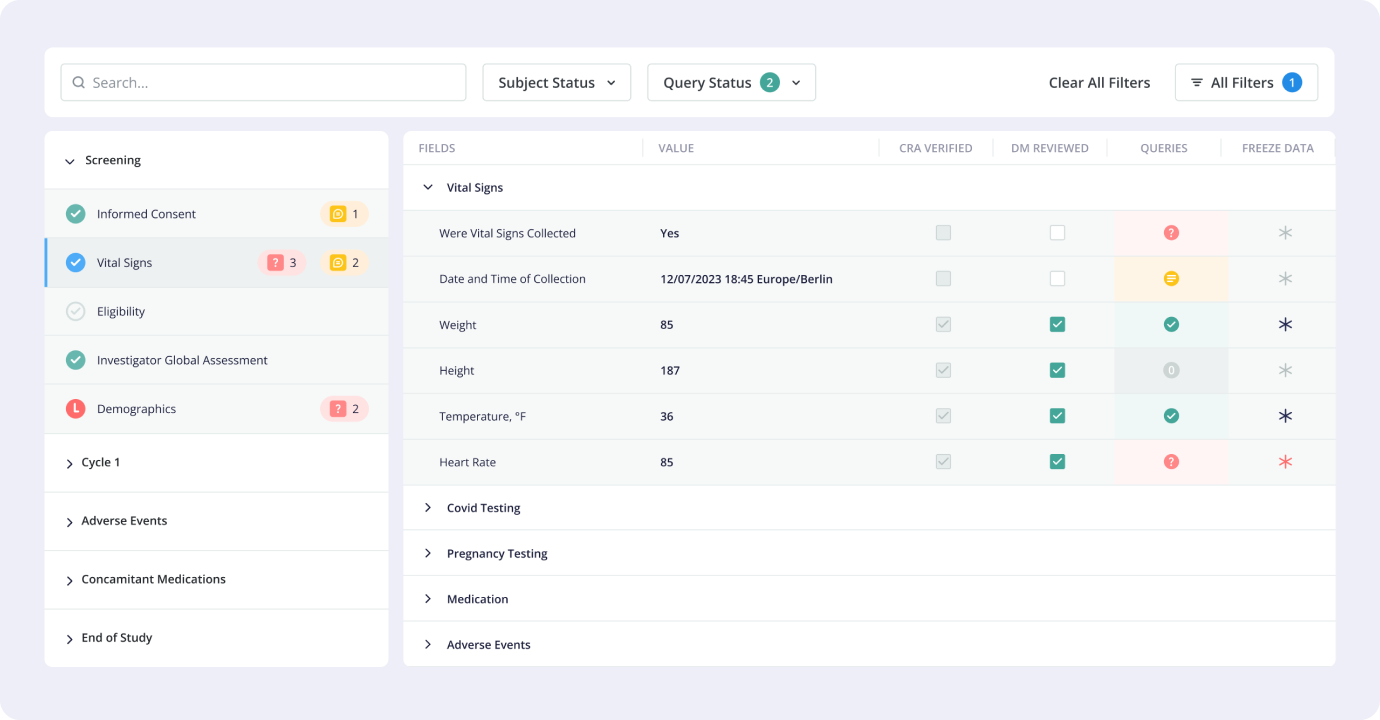
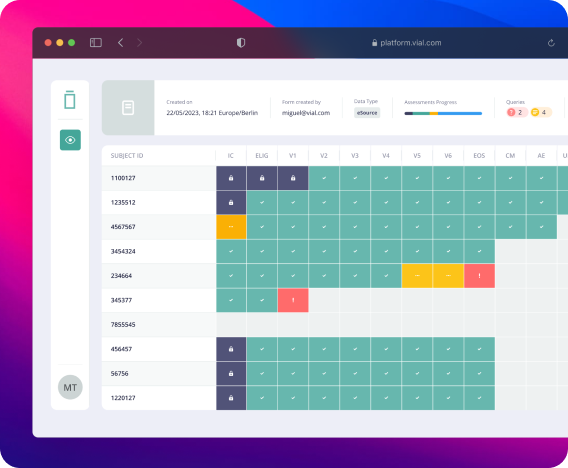
1.5 Results
Vial EDC was
released for production in January 2023, and has been used to efficiently in multiple trials since.
Since release we’ve added more than 20 new functionalities and improvements. Throughout the design
and development process, adherence to regulatory standards was paramount. Compliance with
regulations such as HIPAA, GDPR, and specific clinical trial guidelines ensured data privacy,
security, and integrity.
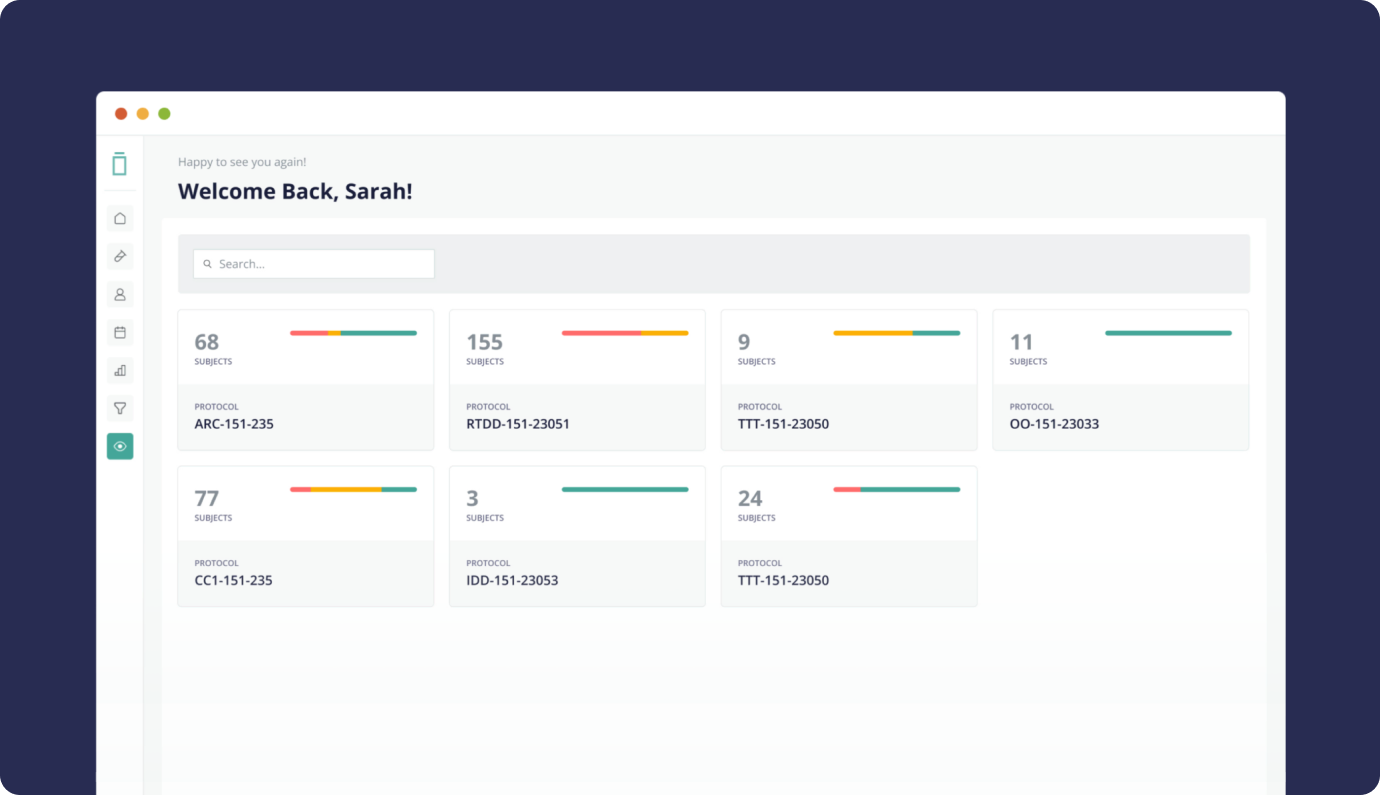
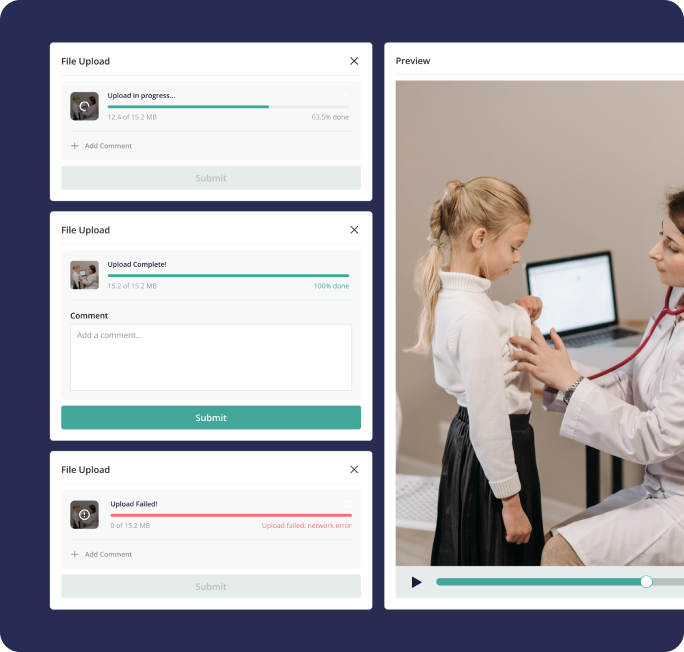
What I've shown above is just a small fraction of the project. We designed entire systems allowing multiple user roles to enter patient data, edit, manage and interact with the data in many different ways. Conducting training sessions and workshops to familiarize users with the system's functionalities, data entry protocols, and best practices, enabling them to navigate the system confidently. By meticulously following the design process steps and engaging stakeholders at every phase, we managed to build an EDC system that delivers a robust, user-friendly, and compliant platform for conducting clinical trials
What I've shown above is just a small fraction of the project. We designed entire systems allowing multiple user roles to enter patient data, edit, manage and interact with the data in many different ways. Conducting training sessions and workshops to familiarize users with the system's functionalities, data entry protocols, and best practices, enabling them to navigate the system confidently. By meticulously following the design process steps and engaging stakeholders at every phase, we managed to build an EDC system that delivers a robust, user-friendly, and compliant platform for conducting clinical trials
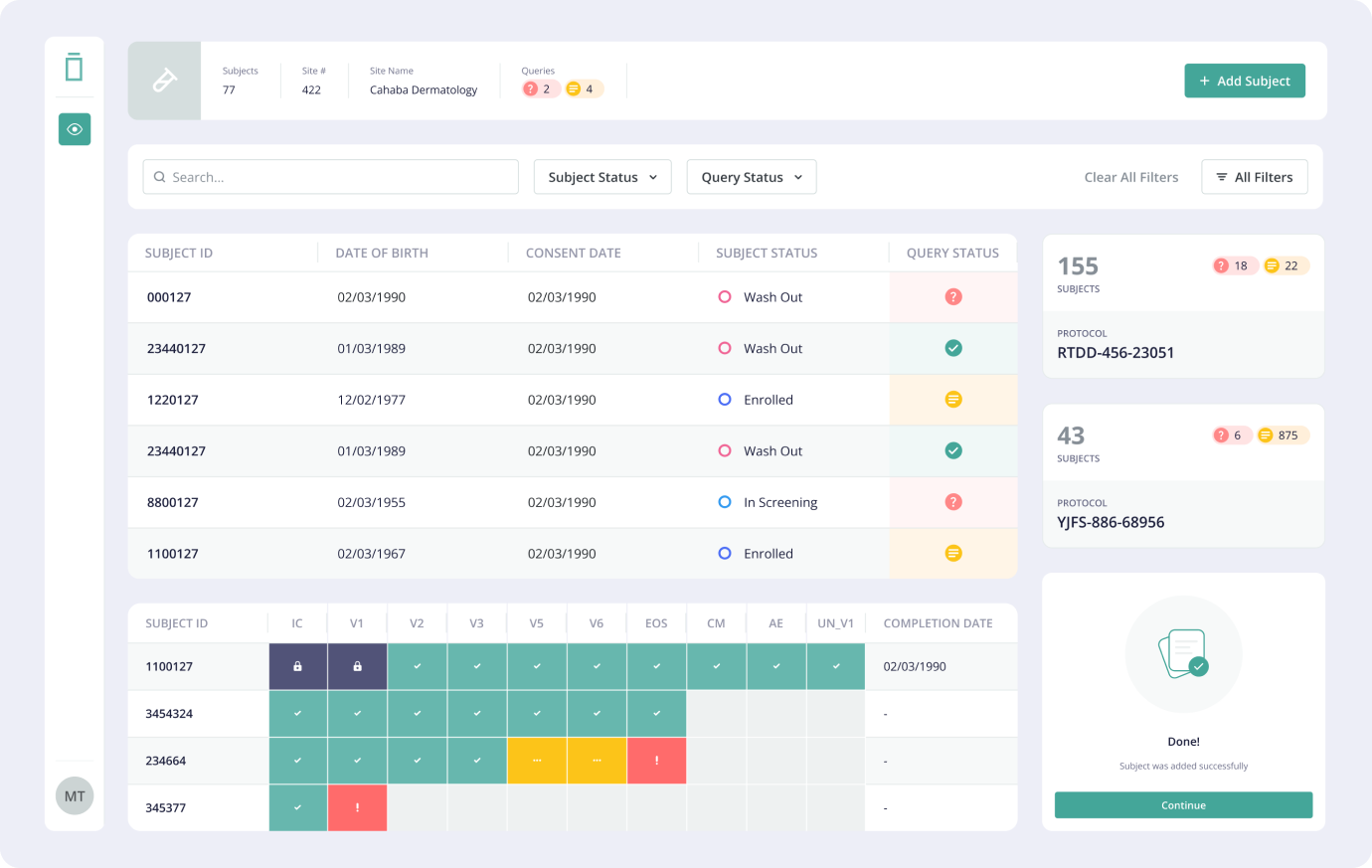
2. Site Startup Platform
Building the Site
Startup Platform was the next step towards enabling faster and more efficient trials for sponsors.
The Site Startup Platform would enable fast and self-service onboarding—entirely digitally for
clinical sites. By eliminating long email threads and archaic document transfer & approval
processes, we would simplify and accelerate site activation. The cloud-based site startup would
enable onboarding for sites in as few as 30 days, as opposed to 90+ days that it normally takes when
done manually.
2.1 Problem Statement and Goals
There is a lack
of a centralized platform for clinical sites to access and manage all outstanding tasks required to
run a trial / study. Sites receive DocuSigns, fillable PDFs, Excel budgets, and requests for
documents, all via email. At any given time, sites are dealing with multiple different trial start
ups and requests for information, with no visibility into completion status.
The current process involves manual tracking of outstanding tasks across multiple platforms (multiple monday boards, email, eTMF) leading to delays in site activation and sponsor timelines.
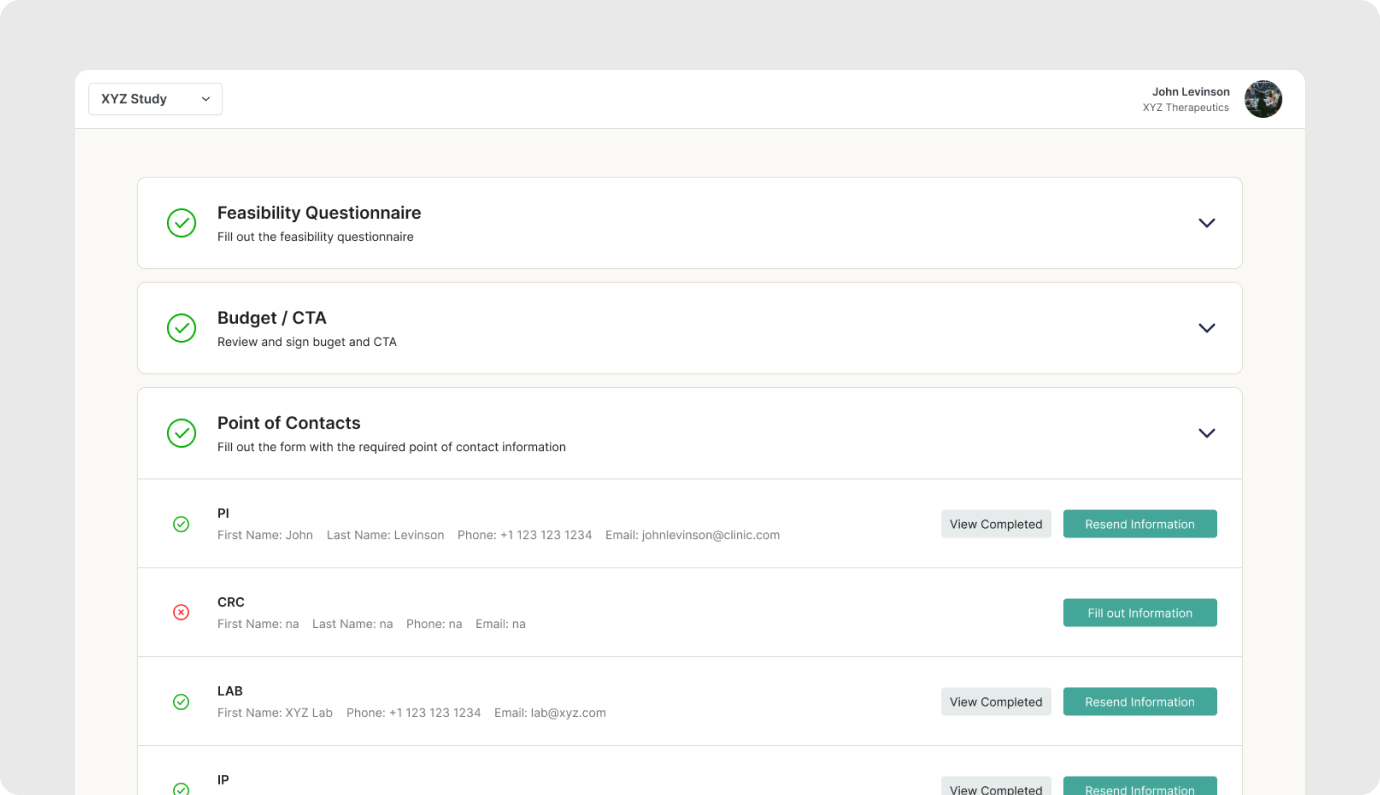
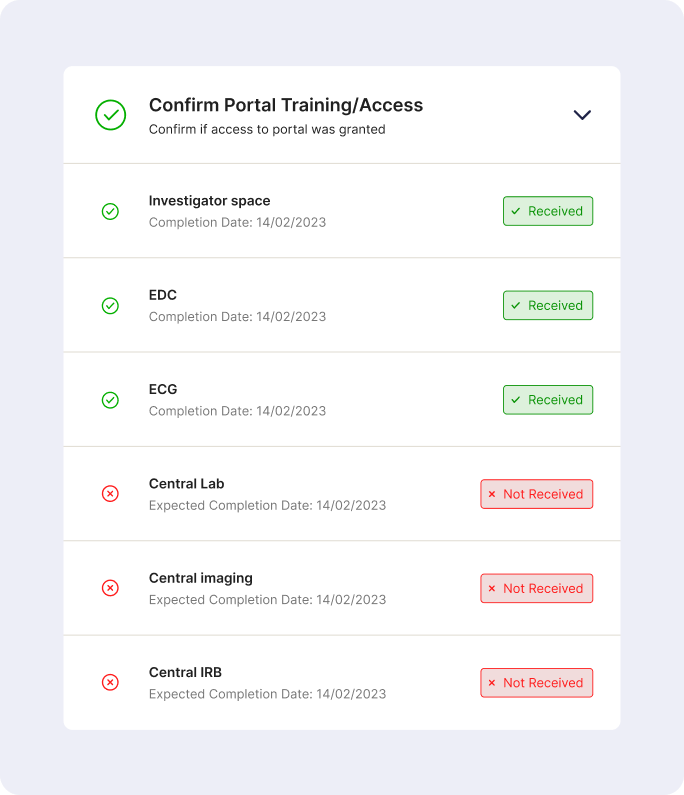
The goal is to provide a unified portal to track individual site onboarding progress, and enable sites to view all outstanding tasks required of them.
The current process involves manual tracking of outstanding tasks across multiple platforms (multiple monday boards, email, eTMF) leading to delays in site activation and sponsor timelines.
The goal is to provide a unified portal to track individual site onboarding progress, and enable sites to view all outstanding tasks required of them.
3. Site Management Platform
In the field of
clinical research operations, effective communication and collaboration between sites and Contract
Research Organizations (CROs) (Vial) is crucial for the success of a study. This time we were
exploring the implementation of a communication and documentation portal to replace email-based
transactional communications. The goal is to centralize key study communications, documents,
milestones, and metrics, while also providing additional features to enhance collaboration and
efficiency.
3.1 Objectives
After rounds of
user interviews and conversations with our clinical operations staff regarding their pain points in
the process of site management we agreed to tackle these 6 objectives first:
1. Eliminate email-based transactional communications between sites and CROs.
1.1 Email communications regarding:
1.1.2 Tasks / To Dos for clinics during a trial
1.1.3 Notification about Upcoming Events; Milestones;
Newsletters; etc
1.1.4 Metrics / KPIs about the study
1.1.5 Regulatory Documents / Training Documents
2. Provide a dedicated portal for sites to access study-related communications,
documents and milestones.
3. Streamline study Key Performance Indicators (KPIs) tracking and reporting.
4. Enable ongoing study task management and notifications.
5. Centralize and ensure up-to-date study documents and protocols.
6. Explore the possibility of integrating a chat bot for easy access to study
information

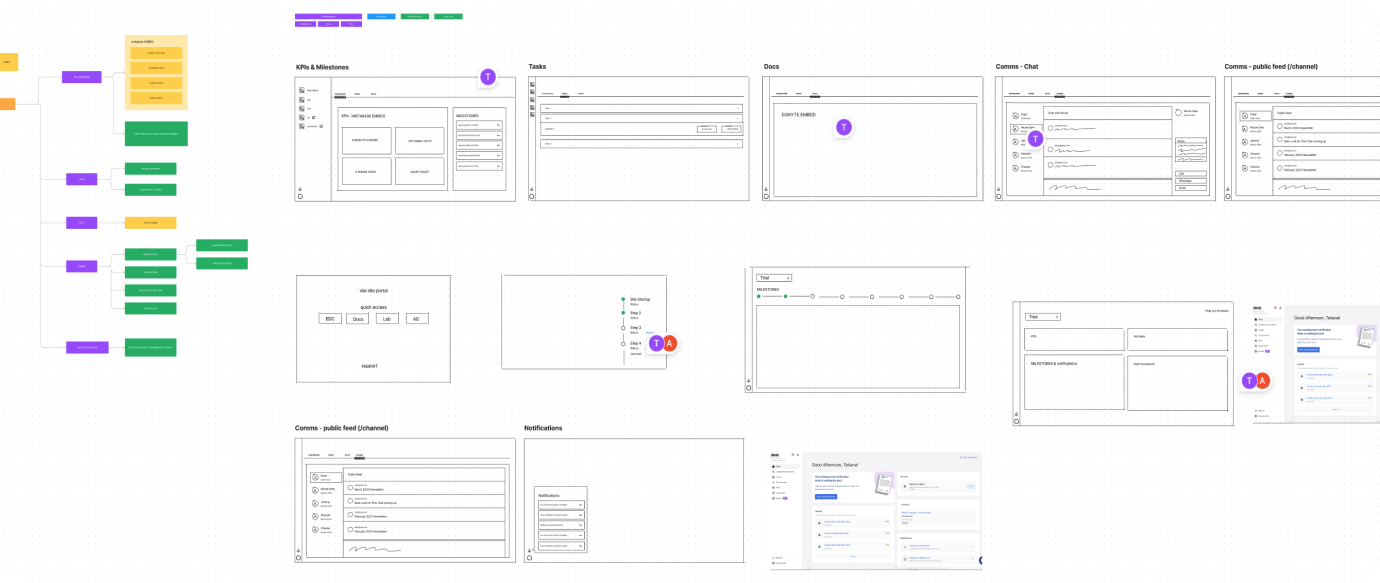
3.2 Solution for a V1
The proposed
solution involves the development and implementation of a communication and information portal for
sites. The portal will serve as a centralized hub for study-related information and facilitate
seamless interactions between sites and Vial.
Key Features:
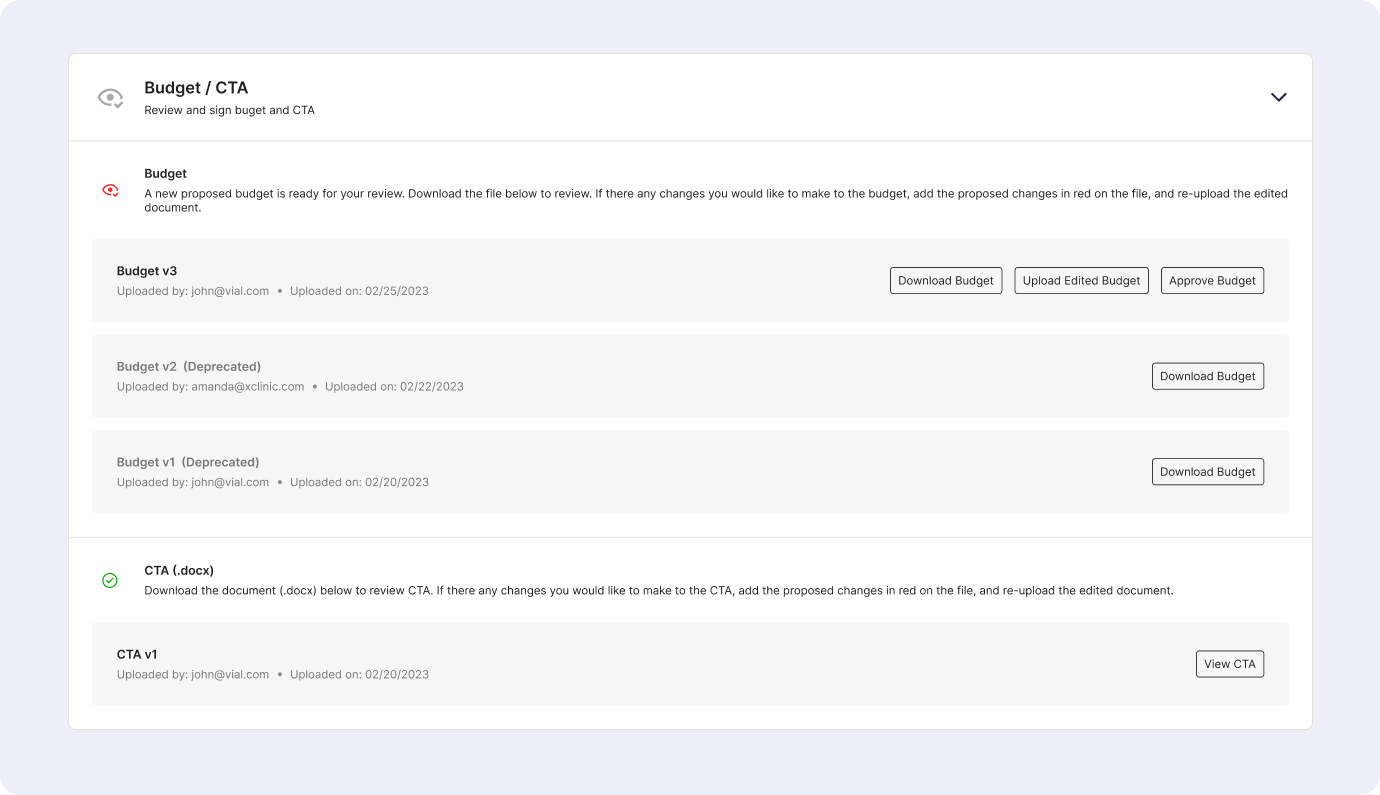
- Quick Access to Trial management apps: The portal will provide links to essential resources, such as the Electronic Data Capture (EDC) system, vendor portals (e.g., 4G, Lab Portal), and Egnyte file storage.
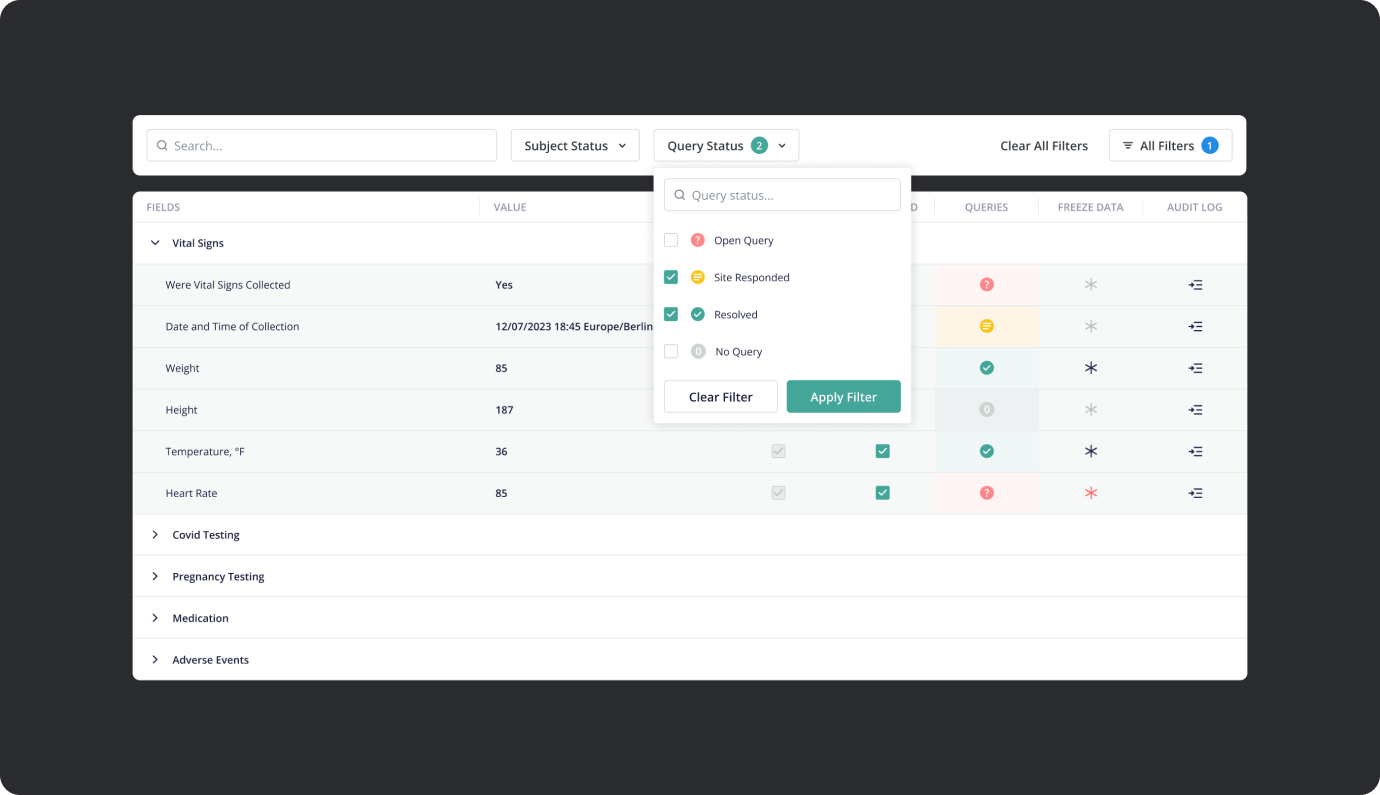
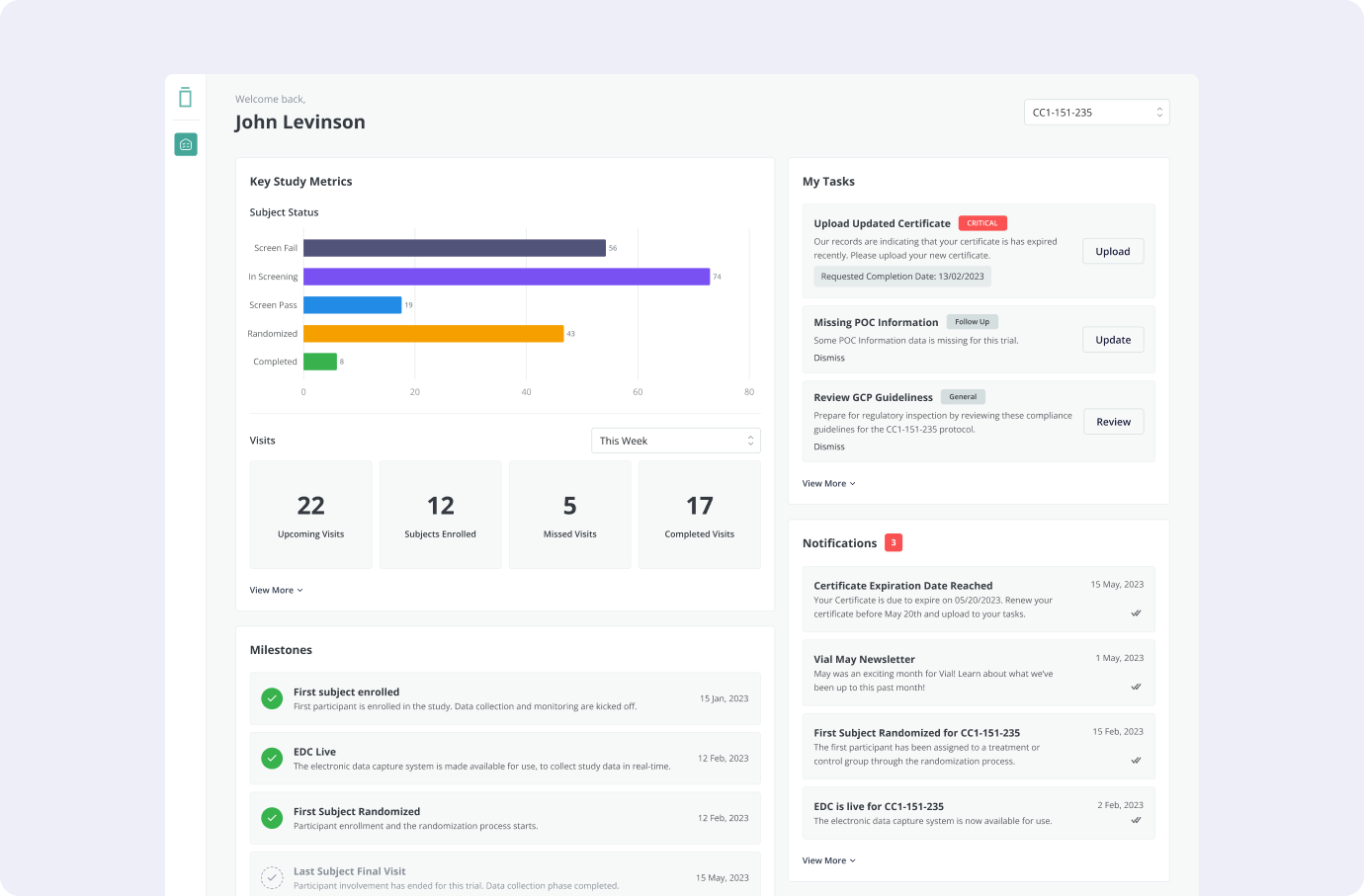
- Study KPIs Tracking: The portal will feature a dashboard displaying study KPIs derived from the EDC system and Monday boards. Examples of tracked metrics include subject status chart, upcoming visits, missed visits count, new query count, and more.
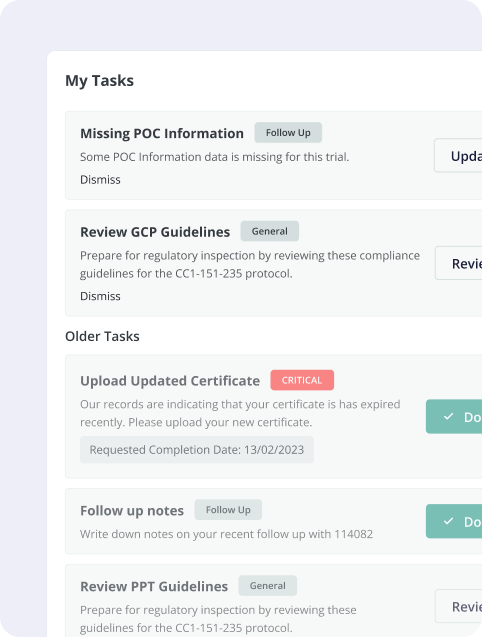
- Ongoing Study Tasks: A dedicated section within the portal will resemble the Site Startup Portal task list. Sites will receive notifications for new tasks, such as uploading updated certificates or submitting specific documentation.
- Storage for Study Documents: The portal will include file views to provide sites with up-to-date study documents, including protocols, guidance documents, and other relevant files.
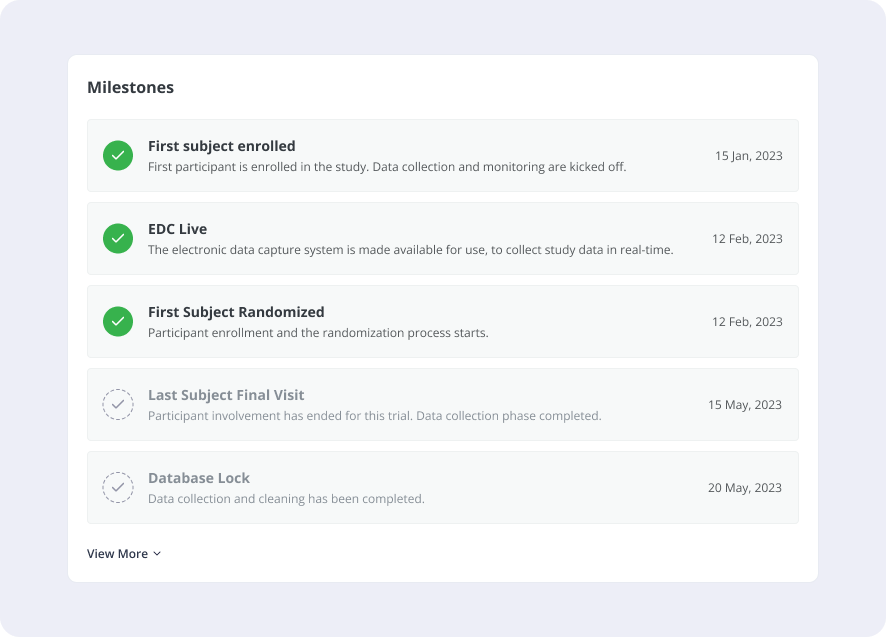
- Notification Center: The portal will incorporate a site-specific and study-wide notification center. Sites will receive notifications regarding upcoming milestones, database lock dates, monitoring visits, and other important study events. For example, before a monitoring visit, the site will be prompted to review specific documents.
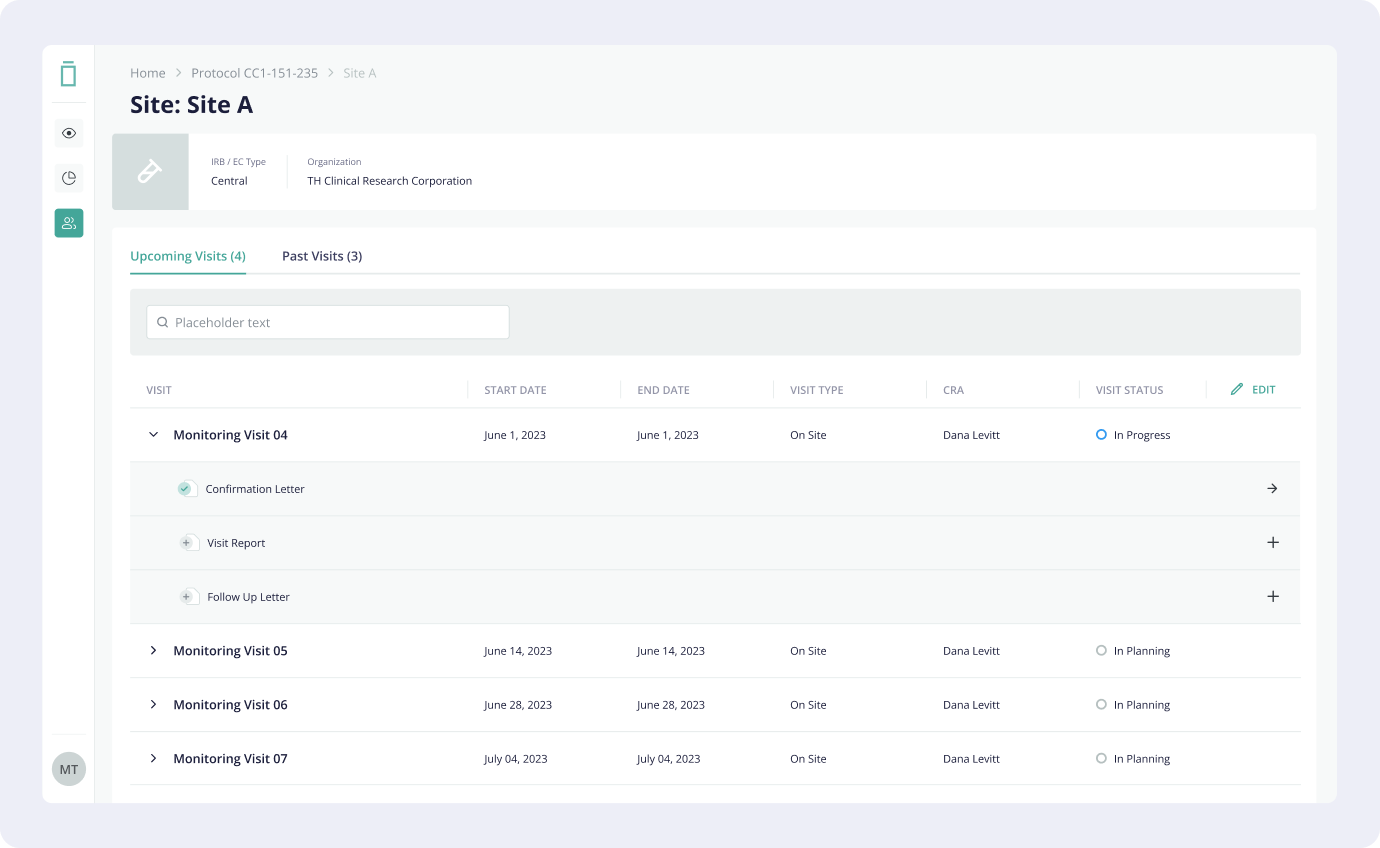
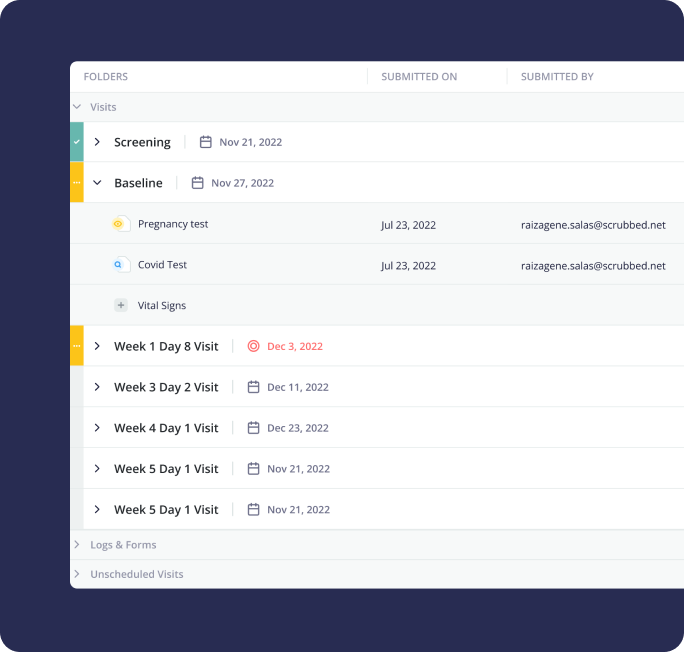
4. Monitoring Portal (CTMS)
Clinical Research
Associates (CRAs) play a pivotal role in clinical trials. They act as liaisons between the sponsor
company and the trial sites conducting the study. Their primary responsibilities include Site
Monitoring and Data Verification. CRAs currently rely on manual processes/personal todo lists to
manage visits, write reports and follow up on action items. By creating a Monitoring Portal that is
integrated with the Electronic Data Capture System (that contains patient data), we would enable
CRAs to spend a lot less hours on visit preparation and closure, leading to more efficiently
conducted monitoring visits.
4.1 Problem Statement
Current CTMS gaps
hinder pace and quality of conducting clinical trials. These include:
- Lack of interoperability: System doesn't work well with other trial systems (EDC, eTMF, IRT), causing data duplication and admin burden.
- Limited customization/automation: Not possible to adjust to study or organization needs, many tasks are manual.
- Limited and manual reporting capabilities: It is hard to document progress or make data driven decisions.
- Risk Based Monitoring: System lacks site performance data, hindering problematic site identification.
- Limited study team collaboration: System lacks good collaboration/doc approval features.

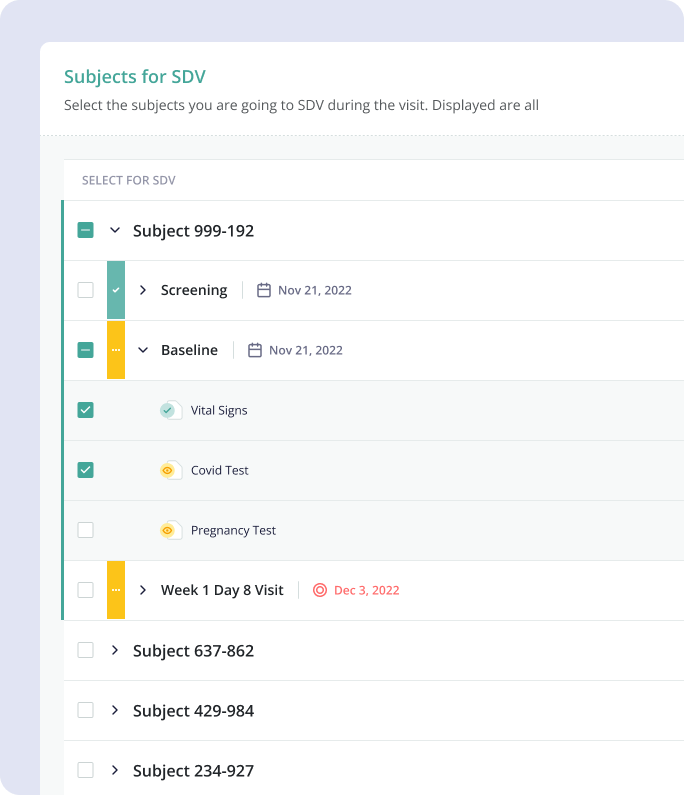
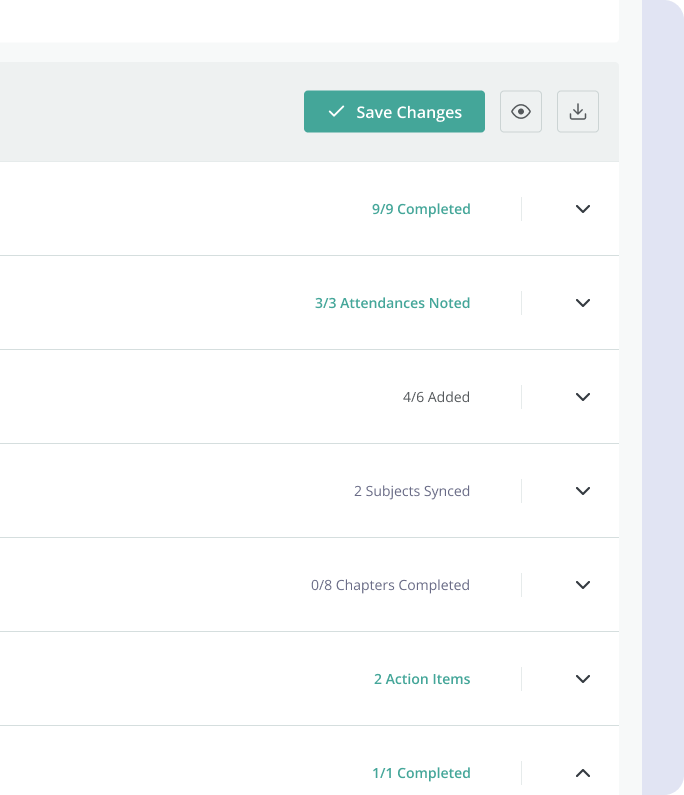
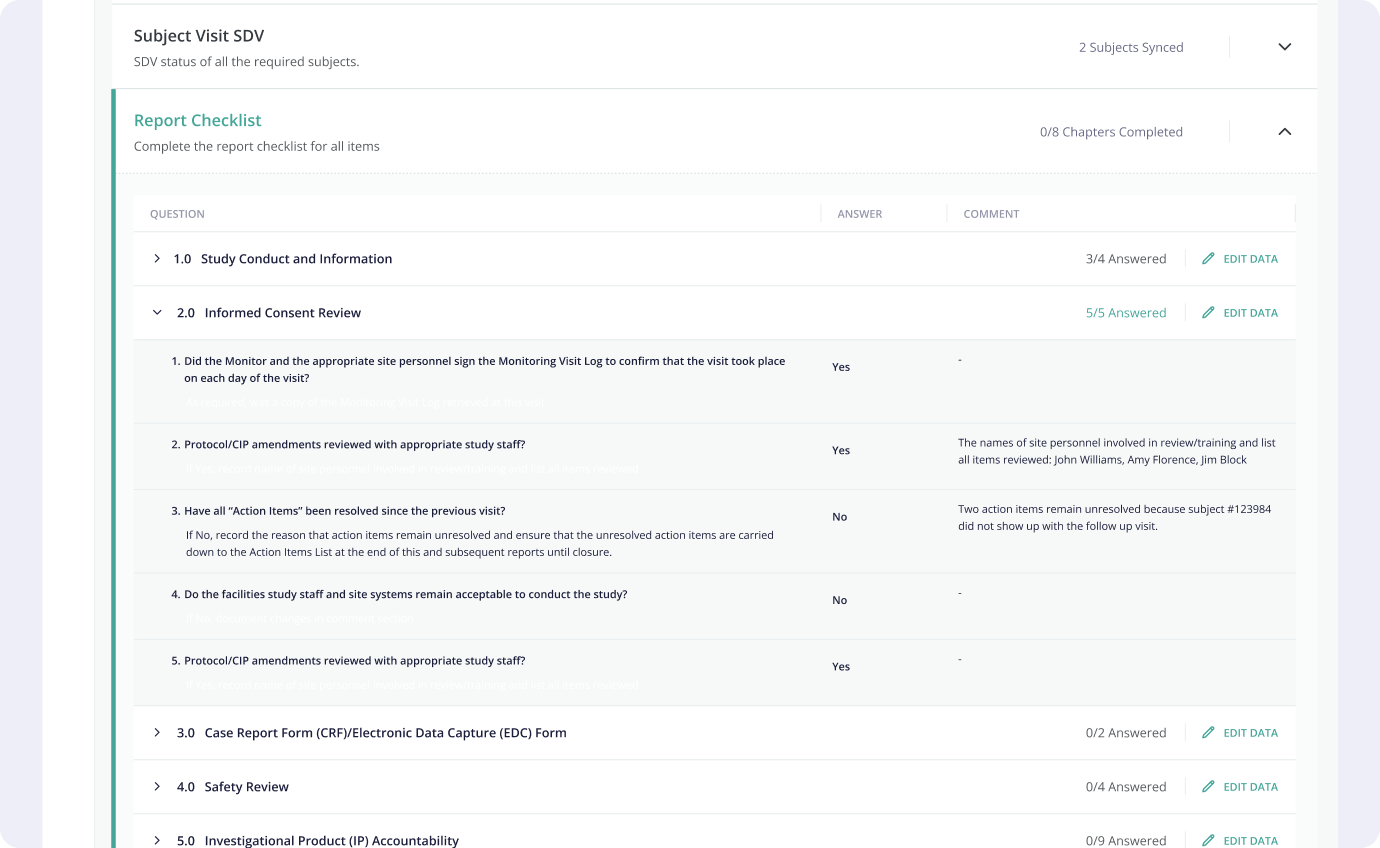
4.2 Solution
The solution
proposed by the design team was an integrated system, allowing CRAs to set up and keep track of
monitoring visits, and create all the necessary reports, where they would be able to keep track of
what data they need to verify, outstanding action items, follow up items, as well as allow
collaboration between users.
Keeping a consistent visual design to the Electronic Data Capture system was crucial in designing the system as users would be expected to switch between the two quite frequently during their visits, so a sense of familiarity with how the system works would be most useful. Another reason for this was to enable the development team to work faster, by reusing elements from other Vial platforms.
The system is currently in development and is expected to be launched in Q1 of 2024.
Keeping a consistent visual design to the Electronic Data Capture system was crucial in designing the system as users would be expected to switch between the two quite frequently during their visits, so a sense of familiarity with how the system works would be most useful. Another reason for this was to enable the development team to work faster, by reusing elements from other Vial platforms.
The system is currently in development and is expected to be launched in Q1 of 2024.